Electronic Clinical Outcome Assessment (eCOA) Solutions Market to hit US$ 7.93 Billion by 2033
eCOA Solutions Market Trends, Growth Drivers & Forecast 2032
United States Electronic Clinical Outcome Assessment Market: Key Trends Reshaping Clinical Trials”
AUSTIN, TX, UNITED STATES, December 15, 2025 /EINPresswire.com/ -- Market Size and Growth— DataM Intelligence
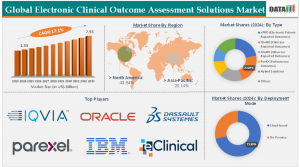
According to DataM Intelligence, Electronic Clinical Outcome Assessment (eCOA) Solutions Market is witnessing rapid expansion, growing from US$ 1.93 Billion in 2024 to US$ 1.67 Billion in 2023, to reach US$ 7.93 Billion by 2033, registering a CAGR of 17.1% during 2025–2032.
The accelerating shift toward decentralized clinical trials, patient-centric study designs, and regulatory mandates for real-time data accuracy is driving adoption of eCOA platforms. By digitizing patient-reported outcomes (PROs), clinician-reported outcomes (ClinROs), observer-reported outcomes (ObsROs), and performance outcomes (PerfOs), eCOA solutions significantly reduce data errors, improve protocol compliance, and enhance trial efficiency across global clinical research ecosystems.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):– https://www.datamintelligence.com/download-sample/electronic-clinical-outcome-assessment-solutions-market
Growth Drivers
1. Over 65% of Phase II–IV clinical trials globally used eCOA tools in 2024, up from 38% in 2020
2. Protocol deviations reduced by up to 45% in trials adopting eCOA versus paper-based assessments
3. Global decentralized and hybrid trials exceeded 12,000 studies in 2024, accelerating digital assessment adoption
4. Regulatory agencies including FDA, EMA, PMDA, and MHRA strongly recommend eCOA for endpoint reliability
5. Pharmaceutical sponsors report average cost savings of USD 2–4 million per large-scale trial using eCOA platforms
Market Segmentation Analysis
By Component
The eCOA market is segmented into Software and Services.
Software dominates with 72% market share (USD 1.73 billion in 2024), driven by cloud-based ePRO platforms, mobile apps, and integrated analytics dashboards. It is projected to reach USD 7.1 billion by 2032 at a 19.8% CAGR.
Services (implementation, validation, training, and support) account for 28% (USD 670 million) and will grow steadily as sponsors and CROs seek end-to-end deployment support.
By Clinical Trial Phase
Phase III trials accounted for 42% of total demand, reflecting large patient populations and complex endpoint tracking
Phase II trials represented 27%, driven by early efficacy and safety validation needs
Phase I and Phase IV trials collectively held 31%, supported by post-marketing surveillance and real-world evidence (RWE) programs
By End User
Pharmaceutical & Biotechnology Companies led the market with 55% share, driven by global pipeline expansion
Contract Research Organizations (CROs) held 30%, supported by outsourcing trends
Academic Research Institutes & Hospitals accounted for 15%, increasingly adopting eCOA for investigator-initiated trials
By Delivery Mode
Cloud-Based eCOA Solutions dominated with 78% share, favored for scalability, global accessibility, and real-time monitoring
On-Premises Solutions accounted for 22%, primarily adopted by organizations with stringent data sovereignty requirements
Application Analysis
1. Oncology represented 30% of total market revenue, driven by complex symptom tracking and long trial duration
2. CNS Disorders accounted for 22%, supported by subjective outcome measures
3. Cardiovascular & Metabolic Diseases held 18%, increasingly using digital PROs
4. Rare Diseases, Immunology, and Others collectively comprised 30%, driven by patient-centric trial designs
Request for Customized Sample Report as per Your Business Requirement:- https://www.datamintelligence.com/customize/electronic-clinical-outcome-assessment-solutions-market
Regional Insights
United States
The U.S. eCOA market was valued at USD 980 million in 2024 and is projected to reach USD 3.9 billion by 2032, growing at a 19.5% CAGR.
Over 70% of FDA-registered trials now mandate digital outcome assessments
Strong presence of global pharma sponsors and CRO headquarters
Widespread adoption of decentralized trial models
Europe
1. Europe accounted for 28% of global market share in 2024.
2. EMA’s emphasis on data integrity and patient-reported outcomes
3. High adoption across Germany, UK, France, and Nordic countries
4. Strong academic and public-funded clinical research ecosystem
Japan
Japan’s eCOA market reached USD 210 million in 2024 and is expected to grow at a 17.8% CAGR through 2032.
PMDA encouragement of electronic endpoints
Rising oncology and CNS clinical trials
Rapid digital transformation of hospital research infrastructure
Asia-Pacific
Asia-Pacific is projected to register the fastest CAGR of 21%, driven by:
Expanding clinical trial activity in China, India, South Korea, and Australia
Cost-efficient patient recruitment and digital health adoption
Increasing multinational trial participation
Key Players
According to DataM Intelligence, the eCOA Solutions Market is moderately consolidated, with technology providers, CRO-backed platforms, and digital health innovators competing on usability, regulatory compliance, and global scalability.
Medidata Solutions | Oracle Health Sciences | Signant Health | Clario | ERT | Kayentis | CRF Health | Suvoda | Castor | QVIA Holdings Inc | Dassault Systems SE | Parexel International Corporation | IBM Corporation | eClinical Solutions LLC | Anju Software
Competitive Highlights
1. Medidata supports over 7,000 clinical trials annually with integrated eCOA platforms
2. IQVIA leverages analytics-driven eCOA for large global trials
3. Signant Health specializes in complex CNS and oncology endpoints
4. Clario integrates eCOA with imaging and biometric data streams
Recent Developments
1. Medidata launched AI-assisted patient engagement features (2025)
2. IQVIA expanded decentralized trial support across Asia-Pacific (2025)
3. Signant Health introduced multilingual eCOA modules for rare disease trials (2024)
4. Oracle Health Sciences enhanced interoperability with EDC and CTMS platforms (2024)
Buy This Report with Year-End Offer (Buy 1 report: Get 30% OFF | Buy 2 reports: Get 50% OFF each! Limited time offer):- https://www.datamintelligence.com/buy-now-page?report=electronic-clinical-outcome-assessment-solutions-market
Market Outlook & Opportunities
1. Global eCOA adoption to exceed 85% of all interventional trials by 2032
2. Oncology and CNS trials to contribute over 50% of total demand
3. Integration with wearables, eConsent, and real-world evidence platforms to unlock new growth
4. AI-driven patient adherence analytics to emerge as a key differentiator
Conclusion
The Electronic Clinical Outcome Assessment (eCOA) Solutions Market is becoming a core pillar of modern clinical research, enabling accurate, patient-centric, and regulatory-compliant outcome measurement. As clinical trials become more decentralized and data-driven, eCOA platforms will play a decisive role in improving trial efficiency, reducing costs, and accelerating drug development timelines through 2032.
Related Report:
Europe Electronic Stethoscope Market
Electronic Stethoscope Market
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.