Clinical Trials Outsourcing Market to Reach USD 101.9 Bn by 2035, Expanding at a CAGR of 6.4% | TMR
Rising cost-efficiency, access to specialized expertise, and the globalization of clinical trials are driving strong market growth worldwide
Growing demand for cost-efficient drug development, access to global patient pools, and specialized expertise is driving expansion in the clinical trials outsourcing market.”
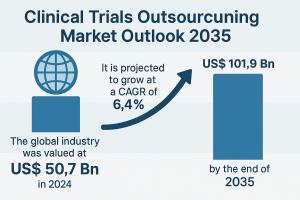
WILMINGTON, DE, UNITED STATES, October 7, 2025 /EINPresswire.com/ -- The global clinical trials outsourcing market is poised for substantial expansion over the next decade. Valued at US$ 50.7 billion in 2024, the market is projected to grow at a CAGR of 6.4% from 2025 to 2035, surpassing US$ 101.9 billion by 2035.— Transparency Market Research
Pharmaceutical, biotechnology, and medical device companies are increasingly outsourcing clinical research operations to contract research organizations (CROs) and specialized service providers to enhance cost efficiency, access expert knowledge, and accelerate drug development timelines. With the growing complexity of clinical trials and the need for global patient diversity, outsourcing has become a critical strategy to ensure data accuracy, regulatory compliance, and faster commercialization of medical innovations.
Full Market Report available for delivery. For purchase or customization, please request here –
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86540
Market Overview
The clinical trials outsourcing market encompasses a wide range of services that include laboratory testing, bioanalytical testing, decentralized clinical trial management, patient recruitment, site identification, data management, logistics, and medical device testing. By outsourcing these services, sponsors can focus on their core research functions while leveraging global expertise and advanced digital infrastructure provided by CROs.
Clinical trial data management services dominate the market, reflecting the rising demand for high-quality, secure, and efficient data handling. With the advent of electronic data capture (EDC) systems, cloud-based platforms, and real-time data monitoring, outsourcing partners have transformed how sponsors collect, analyze, and manage large volumes of clinical data across multiple regions.
Analysts’ Viewpoint
According to analysts at Transparency Market Research, the growing demand for cost-efficient, technology-driven, and globally accessible trial solutions is propelling the expansion of the clinical trials outsourcing market.
Pharmaceutical and biotechnology companies are under increasing pressure to reduce operational costs and accelerate time-to-market for novel therapies. Outsourcing enables access to cutting-edge digital infrastructure, AI-based analytics, and experienced personnel without the overhead burden of maintaining in-house resources.
Leading CROs are embracing artificial intelligence (AI), blockchain, and automation to improve patient recruitment, data monitoring, and protocol design. Partnerships and mergers among CROs and healthcare technology providers are expanding service portfolios and facilitating global reach, allowing companies to execute faster, more reliable, and cost-effective clinical trials.
Clinical Trials Outsourcing Market Introduction
Clinical trials outsourcing refers to delegating various stages of the clinical research process to external organizations specializing in clinical development, regulatory affairs, and data management. Sponsors—ranging from pharmaceutical and biotechnology firms to medical device companies—outsource to achieve operational efficiency, cost reduction, and compliance with global regulatory standards.
Outsourcing allows trial sponsors to focus on innovation and product development, while CROs handle complex logistical operations such as patient enrollment, site management, protocol design, and data analysis. Benefits include accelerated timelines, reduced staffing burdens, and enhanced technological capability. However, maintaining quality control, ensuring data privacy, and managing global regulatory differences remain ongoing challenges.
Key Drivers of Market Growth
1. Growing Cost-efficiency
The rising cost of in-house clinical research is one of the primary factors driving market expansion. Outsourcing helps reduce capital expenses related to infrastructure, staffing, and regulatory compliance. Contracting CROs enables companies—particularly small and mid-sized enterprises—to conduct large-scale trials efficiently and economically.
Outsourcing to geographies with lower operational costs, such as Eastern Europe, Latin America, and Southeast Asia, has become a common strategy. Trials in these regions offer comparable quality at significantly lower costs due to favorable regulations and affordable labor. This geographic flexibility helps sponsors execute cost-effective and scalable clinical trials without compromising data integrity.
2. Access to Specialized Expertise and Global Patient Populations
Access to expert talent and diverse patient populations significantly enhances trial success rates. CROs possess specialized teams, advanced equipment, and years of regulatory experience, ensuring efficient trial execution in line with global standards.
Outsourcing enables sponsors to reach diverse patient groups across multiple continents, accelerating patient recruitment and improving data representativeness. For rare or orphan diseases, CROs’ global reach and therapeutic expertise are crucial for identifying eligible patients and delivering statistically robust outcomes.
3. Technological Advancements Driving Operational Efficiency
The integration of AI, IoT, and cloud computing has revolutionized the clinical trial landscape. Real-time monitoring, predictive analytics, and decentralized trial models are now essential features in outsourced clinical research. These advancements reduce data errors, improve compliance, and enhance trial transparency.
Digital tools such as EDC platforms, remote patient monitoring, and eConsent systems have further streamlined trial management and improved patient engagement, making outsourcing an indispensable model for modern clinical development.
Segment Analysis
By Service Type
Clinical Trial Data Management Services lead the global market, supported by growing trial complexity and the need for secure, real-time data handling.
Other key services include laboratory testing, bioanalytical testing, decentralized clinical trial services, patient recruitment, analytical testing, logistics, and medical device testing.
By Therapeutic Area
The market spans oncology, infectious diseases, neurology, metabolic disorders, immunology, cardiology, genetic diseases, women’s health, and others—each driving demand for customized CRO capabilities and therapeutic expertise.
By End User
Pharmaceutical and biotechnology companies account for the largest share, followed by medical device manufacturers seeking regulatory support and specialized testing capabilities.
Regional Outlook
North America dominates the global clinical trials outsourcing market, driven by advanced healthcare infrastructure, technological leadership, and strong regulatory frameworks led by the U.S. Food and Drug Administration (FDA). The region’s diverse patient pool and well-established CRO ecosystem accelerate trial timelines and ensure compliance.
Europe represents another major hub, with the U.K., Germany, and France leading in clinical research activity. The region’s stringent quality standards and investment in digital health technologies further enhance market performance.
Asia Pacific is expected to witness the fastest growth, supported by expanding patient populations, lower operational costs, and increasing participation in global clinical trials. Countries such as China, India, South Korea, and Japan are emerging as key outsourcing destinations.
Latin America and the Middle East & Africa are gaining traction due to improving healthcare infrastructure and favorable government initiatives, though awareness and regulatory harmonization remain areas for development.
Key Players and Industry Leaders
The global clinical trials outsourcing market is characterized by the presence of leading CROs and service providers focused on innovation, data integrity, and global expansion.
Prominent players include:
ICON plc, IQVIA, Thermo Fisher Scientific Inc., SGS Life Sciences, Charles River Laboratories, Parexel, Syneos Health, Medpace, LabCorp, KCR, PRA Health Sciences, WuXi AppTec, Pharmaron, and Avance Clinical.
These companies are strengthening service portfolios through mergers, acquisitions, and AI integration, enhancing trial efficiency and reliability.
Recent Developments
January 2025 – ICON plc: Expanded its AI portfolio to improve clinical trial efficiency through new automation and predictive analytics tools, including iSubmit, FORWARD+, and OMR AI Navigation Assistant.
June 2024 – IQVIA: Introduced One Home for Sites, a digital platform designed to simplify technology use and data flow for clinical research sites.
Opportunities and Challenges
Opportunities:
Rapid adoption of digital and decentralized trial technologies
Expansion into emerging economies with diverse patient pools
Growth in biologics, gene therapy, and personalized medicine
Increased partnerships between CROs and biotech startups
Challenges:
Data security and confidentiality risks
Complex global regulatory environments
High competition among CROs and margin pressures
Market Trends
Decentralized Clinical Trials (DCTs): Enhanced flexibility through remote participation and telemedicine.
AI and Automation: Streamlining data analytics, patient matching, and trial forecasting.
Cloud-based and Real-time Data Systems: Enabling faster decision-making and improved data transparency.
Collaborative CRO Ecosystem: Rising partnerships for cross-regional expertise and integrated services.
Future Outlook
The clinical trials outsourcing market is set for robust growth through 2035, driven by digital transformation, global collaboration, and the rising need for efficient drug development processes. Technological innovations, coupled with regulatory harmonization and patient-centric trial models, will further accelerate market expansion.
Companies investing in AI-enabled, cost-effective, and globally connected trial solutions are likely to capture significant market share in the coming decade.
Why Buy This Report?
Comprehensive market forecasts and CAGR analysis through 2035
In-depth insights into key growth drivers, restraints, and opportunities
Detailed segmentation by service type, therapeutic area, end user, and region
Profiles of major market players with strategic initiatives and recent developments
Evaluation of emerging trends, digital transformation, and regional growth patterns
More Related Reports-
Clinical Trials Market - https://www.transparencymarketresearch.com/clinical-trials-market.html
HCRO Market (Healthcare Contract Research Outsourcing) - https://www.transparencymarketresearch.com/hcro-market.html
Medical Affairs Outsourcing Market - https://www.transparencymarketresearch.com/medical-affairs-outsourcing-market.html
Contract Research Organizations Services Market - https://www.transparencymarketresearch.com/contract-research-organizations-services-market.html
About Us Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
Contact Us
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Atil Chaudhari
Transparency Market Research Inc.
+1 518-618-1030
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.