Advanced Liver Cancer Clinical Trial Pipeline Shows Potential with Active Contributions from 50+ Key Companies | DelveInsight
The advanced liver cancer market is positioned for strong growth as rising incidence rates, driven by chronic liver diseases and metabolic disorders, expand the patient pool and boost demand for effective therapies. Increasing adoption of screening programs supports earlier diagnosis, while the launch of innovative targeted therapies and immuno-oncology combinations is transforming the treatment landscape and offering significant commercial opportunities. Together, these factors create a favorable environment for sustained market expansion, despite challenges such as high treatment costs and access disparities in some regions.
New York, USA, Sept. 25, 2025 (GLOBE NEWSWIRE) -- Advanced Liver Cancer Clinical Trial Pipeline Shows Potential with Active Contributions from 50+ Key Companies | DelveInsight
The advanced liver cancer market is positioned for strong growth as rising incidence rates, driven by chronic liver diseases and metabolic disorders, expand the patient pool and boost demand for effective therapies. Increasing adoption of screening programs supports earlier diagnosis, while the launch of innovative targeted therapies and immuno-oncology combinations is transforming the treatment landscape and offering significant commercial opportunities. Together, these factors create a favorable environment for sustained market expansion, despite challenges such as high treatment costs and access disparities in some regions.
DelveInsight’s 'Advanced Liver Cancer Pipeline Insight 2025' report provides comprehensive global coverage of pipeline advanced liver cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the advanced liver cancer pipeline domain.
Key Takeaways from the Advanced Liver Cancer Pipeline Report
- DelveInsight’s advanced liver cancer pipeline report depicts a robust space with 50+ active players working to develop 52+ pipeline advanced liver cancer drugs.
- Key advanced liver cancer companies such as Polaris Pharmaceuticals, Shanghai Henlius Biotech, Tyra Biosciences, Tvardi Therapeutics, Jiangsu Hengrui Pharmaceuticals Co., Ltd., TransThera Sciences, Zhejiang Haichang Biotech Co., Ltd., AstraZeneca, Ascentawits Pharmaceuticals, Ltd, Qurient Co., Ltd., TORL Biotherapeutics, Aptamer Sciences, Inc., Qurgen Inc., Zymeworks BC Inc., Ltd., Qurient Co., Ltd., Myeloid Therapeutics, BioInvent International AB, GV20 Therapeutics, Etnova Therapeutics Corp., and others are evaluating new advanced liver cancer drugs to improve the treatment landscape.
- Promising pipeline advanced liver cancer therapies, such as Pegargiminase, HLX43, TYRA-430, TTI-101, SHR-8068, TT-00420, WGI-0301, Volrustomig, AST-3424, Q702, TORL-4-500, AST-201, SON-DP, ZW251, Q702, MT-303, BI-1910, GV20-0251, ETN101, and others, are in different phases of advanced liver cancer clinical trials.
- In June 2025, Tempest Therapeutics, Inc. announced that the company received approval from the National Medical Products Administration (NMPA) in China to proceed with a pivotal trial to evaluate amezalpat (TPST-1120) in combination with atezolizumab and bevacizumab, the current standard of care, versus the standard of care alone in the first-line treatment of patients with unresectable or metastatic hepatocellular carcinoma (HCC).
- In June 2025, Tempest Therapeutics, Inc. announced that the European Medicines Agency (EMA) had granted Orphan Drug Designation (ODD) to amezalpat (TPST-1120), an oral, small molecule, selective PPAR⍺ antagonist for the treatment of patients with hepatocellular carcinoma (HCC).
- In April 2025, Bayer announced the initiation of a Phase I clinical trial with 225Ac-GPC3 (BAY 3547926), an investigational targeted alpha radiopharmaceutical being developed to treat tumors expressing Glypican-3 (GPC3) in patients with advanced hepatocellular carcinoma (HCC).
- In February 2025, Tempest Therapeutics, Inc. announced that the FDA has granted amezalpat, a small molecule, oral, selective PPAR⍺ antagonist, fast track designation for the treatment of patients with HCC.
- In January 2025, Tempest Therapeutics, Inc. announced that the US Food and Drug Administration (FDA) had granted Orphan Drug Designation (ODD) to amezalpat (TPST-1120), an oral, small molecule, selective PPAR⍺ antagonist for the treatment of patients with hepatocellular carcinoma (HCC).
- In January 2025, NiKang Therapeutics® Inc. announced that the first patient had been dosed in the global randomized phase Ib/II clinical study evaluating NKT2152, a highly potent, selective, and orally bioavailable small molecule HIF2α inhibitor, in combination with a standard-of-care regimen of atezolizumab (Tecentriq®) and bevacizumab (Avastin®) in the first-line treatment of patients with advanced or metastatic HCC.
- In October 2024, Tempest Therapeutics, Inc. announced an agreement with Roche to advance the evaluation of amezalpat (TPST-1120) in combination with atezolizumab (Tecentriq®) and bevacizumab, the current standard of care for unresectable or metastatic hepatocellular carcinoma (HCC), into a pivotal Phase III trial for the first-line treatment of unresectable or metastatic hepatocellular carcinoma, a form of liver cancer with high unmet need.
- In July 2024, Myeloid Therapeutics, Inc. dosed the first patient with MT-303 in a Phase I study for hepatocellular carcinoma (HCC). MT-303 is Myeloid's second in vivo mRNA CAR program to enter the clinic from its pipeline of in vivo immune cell programming therapies. Dosing with MT-303 represents a significant milestone in bringing advanced novel therapies to people with liver cancer.
- In June 2024, AbelZeta Pharma, Inc. announced preliminary safety and efficacy results from its first-time in human investigator-initiated trial (IIT) of C-CAR031. The presentation shared data indicating a manageable safety profile and encouraging anti-tumor activity of C-CAR031 in patients with heavily pretreated advanced hepatocellular carcinoma (HCC).
Request a sample and discover the recent advances in advanced liver cancer drugs @ Advanced Liver Cancer Pipeline Report
The advanced liver cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage advanced liver cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the advanced liver cancer clinical trial landscape.
Advanced Liver Cancer Overview
Advanced liver cancer represents a progressed stage of hepatic malignancy in which the cancer has extended beyond the liver to nearby lymph nodes (stage 4A) or distant organs like the lungs or bones (stage 4B), making surgical removal no longer a viable option. Patients commonly experience symptoms such as jaundice, abdominal pain, fatigue, unintended weight loss, and other systemic manifestations.
Management at this stage focuses on slowing the progression of the tumor, reducing symptoms, and maintaining quality of life through the use of immunotherapy, targeted treatments, transarterial chemoembolization (TACE), radiotherapy, or chemotherapy. Supportive care is fundamental for controlling complications like ascites, hepatic encephalopathy, and pain, which have a substantial impact on patient comfort. Despite ongoing advancements in systemic therapy, the prognosis remains poor, with median survival in metastatic disease often limited to just a few months.
Diagnosis of advanced liver cancer involves a comprehensive, multidisciplinary approach that combines imaging, laboratory tests, and, in select cases, biopsy to confirm the disease, determine stage, and assess spread. High-resolution imaging modalities such as contrast-enhanced CT and MRI are critical for identifying primary tumors and evaluating their spread to lymph nodes or distant organs. PET or bone scans may be used to detect metastases outside the liver. While ultrasound remains helpful for early-stage detection, it has limited sensitivity in advanced stages.
Blood tests, including alpha-fetoprotein (AFP) and the AFP-L3 isoform, support diagnosis, especially when aligned with imaging findings. Liver function tests help assess hepatic reserve and the tumor’s impact on organ function. Biopsies are generally reserved for cases where imaging is inconclusive or when histological confirmation is needed for atypical lesions or distant metastases. Staging of advanced disease often includes chest imaging, bone scans, and occasionally brain imaging to guide therapeutic decisions. Although imaging plays a central role, diagnostic accuracy is enhanced when combined with serological markers and the clinical picture, allowing for individualized treatment planning.
Treatment strategies for advanced liver cancer focus on slowing disease progression, relieving symptoms, and prolonging life. Immunotherapy has become a primary option, with regimens such as atezolizumab plus bevacizumab or nivolumab plus ipilimumab offering meaningful survival advantages by stimulating the immune system to target cancer cells.
Targeted therapies, which interfere with key molecular pathways in tumor growth, are used either when immunotherapy is unsuitable or after disease progression. Radiation therapy, particularly stereotactic body radiotherapy (SBRT), helps control localized tumor burden and manage symptoms such as pain from bone metastases. In patients whose disease is still confined to the liver and who retain good liver function, procedures like TACE can still provide clinical benefit. Chemotherapy is used less frequently but may be employed in resistant or refractory cases. Throughout the course of the illness, supportive care remains crucial, addressing pain management, nutritional support, fluid control, and psychosocial needs.
Find out more about advanced liver cancer drugs @ Advanced Liver Cancer Treatment
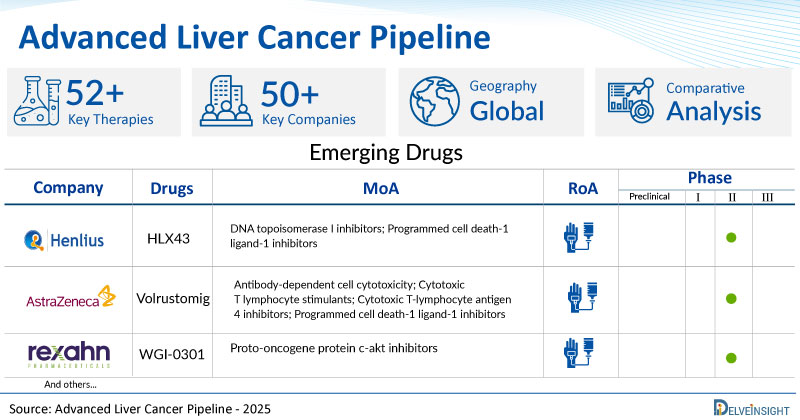
A snapshot of the Pipeline Advanced Liver Cancer Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| SHR-8068 | Suzhou Suncadia Biopharmaceuticals | III | Antibody-dependent cell cytotoxicity; Cytotoxic T-lymphocyte antigen 4 modulators; T lymphocyte stimulants | Intravenous |
| HLX43 | Shanghai Henlius Biotech | II | DNA topoisomerase I inhibitors; Programmed cell death-1 ligand-1 inhibitors | Intravenous |
| Volrustomig | AstraZeneca | II | Antibody-dependent cell cytotoxicity; Cytotoxic T lymphocyte stimulants; Cytotoxic T-lymphocyte antigen 4 inhibitors; Programmed cell death-1 ligand-1 inhibitors | Intravenous |
| WGI-0301 | Rexahn Pharmaceuticals | II | Proto-oncogene protein c-akt inhibitors | Intravenous |
| TT-00420 | TransThera Biosciences | II | Aurora kinase A inhibitors; Aurora kinase B inhibitors; Fibroblast growth factor receptor antagonists; Janus kinase inhibitors; Vascular endothelial growth factor receptor antagonists | Oral |
| TTI-101 | Tvardi Therapeutics | I/II | STAT3 transcription factor inhibitors | Oral |
| AST-3424 | Ascentawits Pharmaceuticals, Ltd | I/II | Alkylating agents; DNA cross linking agents | Intravenous |
| TYRA-430 | Tyra Biosciences | I | Type 3 fibroblast growth factor receptor antagonists; Type 4 fibroblast growth factor receptor antagonists | Oral |
Learn more about the emerging advanced liver cancer therapies @ Advanced Liver Cancer Clinical Trials
Advanced Liver Cancer Therapeutics Assessment
The advanced liver cancer pipeline report proffers an integral view of the emerging advanced liver cancer therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Advanced Liver Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Antibody-dependent cell cytotoxicity; Cytotoxic T-lymphocyte antigen 4 modulators; T lymphocyte stimulants, DNA topoisomerase I inhibitors; Programmed cell death-1 ligand-1 inhibitors, Programmed cell death-1 ligand-1 inhibitors, Proto-oncogene protein c-akt inhibitors, Aurora kinase A inhibitors; Aurora kinase B inhibitors; Fibroblast growth factor receptor antagonists; Janus kinase inhibitors; Vascular endothelial growth factor receptor antagonists, STAT3 transcription factor inhibitors, Alkylating agents; DNA cross linking agents, Type 3 fibroblast growth factor receptor antagonists and Type 4 fibroblast growth factor receptor antagonists.
- Key Advanced Liver Cancer Companies: Polaris Pharmaceuticals, Shanghai Henlius Biotech, Tyra Biosciences, Tvardi Therapeutics, Jiangsu Hengrui Pharmaceuticals Co., Ltd., TransThera Sciences, Zhejiang Haichang Biotech Co., Ltd., AstraZeneca, Ascentawits Pharmaceuticals, Ltd, Qurient Co., Ltd., TORL Biotherapeutics, Aptamer Sciences, Inc., Qurgen Inc., Zymeworks BC Inc., Ltd., Qurient Co., Ltd., Myeloid Therapeutics, BioInvent International AB, GV20 Therapeutics, Etnova Therapeutics Corp., and others.
- Key Advanced Liver Cancer Pipeline Therapies: Pegargiminase, HLX43, TYRA-430, TTI-101, SHR-8068, TT-00420, WGI-0301, Volrustomig, AST-3424, Q702, TORL-4-500, AST-201, SON-DP, ZW251, Q702, MT-303, BI-1910, GV20-0251, ETN101, and others.
Dive deep into rich insights for new advanced liver cancer treatments, visit @ Advanced Liver Cancer Drugs
Table of Contents
| 1. | Advanced Liver Cancer Pipeline Report Introduction |
| 2. | Advanced Liver Cancer Pipeline Report Executive Summary |
| 3. | Advanced Liver Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Advanced Liver Cancer Clinical Trial Therapeutics |
| 6. | Advanced Liver Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Advanced Liver Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | Advanced Liver Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | Advanced Liver Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | Advanced Liver Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Advanced Liver Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Advanced Liver Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the advanced liver cancer pipeline therapeutics, reach out @ Advanced Liver Cancer Therapeutics
Related Reports
Advanced Liver Cancer Epidemiology Forecast
Advanced Liver Cancer Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted advanced liver cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Advanced Liver Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key advanced liver cancer companies, including Binhui Biopharmaceutical Co. Ltd., Hoffmann-La Roche, Adagene Inc., Tempest Therapeutics, Abbisko Therapeutics Co. Ltd., Qilu Pharmaceutical Co Ltd., Mina Alpha Limited, Jiangsu Hengrui Pharmaceutical Co., Ltd., Daiichi Sankyo Inc., Sanofi, Roche Pharma AG, Jennerex Biotherapeutics, Mayo Clinic, Bristol-Myers Squibb, Eli Lilly and Company, among others.
Liver Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key liver cancer companies, including Arcus Biosciences, Yiviva, Virogin Biotech, Tvardi Therapeutics, GlaxoSmithKline, TORL Biotherapeutics, AVEO Pharmaceuticals, Teclison, Epizyme, Sirnaomics, Coherus Biosciences, Sinocelltech Ltd., Qurient Co., Hoffmann-La Roche, Can-Fite BioPharma, Omega Therapeutics, Novita Pharmaceuticals, Bristol-Myers Squibb, among others.
Liver Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key liver cancer companies, including Arcus Biosciences, Yiviva, Virogin Biotech, Tvardi Therapeutics, GlaxoSmithKline, TORL Biotherapeutics, AVEO Pharmaceuticals, Teclison, Epizyme, Sirnaomics, Coherus Biosciences, Sinocelltech Ltd., Qurient Co., Hoffmann-La Roche, Can-Fite BioPharma, Omega Therapeutics, Novita Pharmaceuticals, Bristol-Myers Squibb, among others.
Metastatic Liver Cancer Pipeline
Metastatic Liver Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key metastatic liver cancer companies, including Oncorus, Sirnaomics, Codiak BioSciences, Sorrento Therapeutics, Inc., RemeGen Co., Ltd., Taiho Pharmaceutical Co., Ltd., Amarin Corporation, Eureka Therapeutics, Medivir AB, AstraZeneca, Amal Therapeutics, Boehringer Ingelheim, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
