Virtual Clinical Trials Market Expands as Pharma Embraces Digital Research $14.42B by 2033 | DataM Intelligence
Virtual Clinical Trials market grows to $14.42B by 2033, driven by digital health, patient-centric models & AI-powered decentralized research.
By 2033, the $14.42B Virtual Clinical Trials market will redefine patient access, speed & efficiency in global drug R&D.”
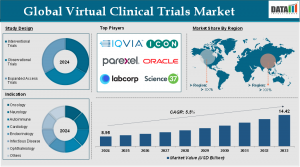
AUSTIN, TX, UNITED STATES, June 19, 2025 /EINPresswire.com/ -- The Virtual Clinical Trials Market reached US$ 8.95 billion in 2024 and is expected to reach US$ 14.42 billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025–2033. This strong growth underscores how rapidly the clinical research world is shifting toward decentralized and patient-centric models powered by advancements in digital health and real-time data capture.— DataM Intelligence
Traditional site-based clinical trials are becoming increasingly complex and expensive, with challenges in patient recruitment, retention, and operational efficiency. Virtual clinical trials, or VCTs, address many of these hurdles by allowing patients to participate remotely, leveraging telemedicine, mobile apps, wearable devices, e-consent platforms, and cloud-based data systems.
To Download Sample Report: https://datamintelligence.com/download-sample/virtual-clinical-trials-market
Key Growth Drivers
1. Patient-Centric Approaches:
Patients today demand flexibility. Virtual trials offer convenience by minimizing site visits, allowing participation from home, and enabling broader geographic access. This improves enrollment and retention rates critical factors in trial success.
2. Technology Maturation:
Advances in telemedicine, wearables, and AI-powered analytics have made remote monitoring feasible and reliable. Real-time patient data is now collected with high precision, helping researchers make faster and more informed decisions.
3. Regulatory Support:
Global regulators, including the FDA, EMA, and Japanese PMDA, increasingly endorse hybrid and virtual models. They’ve issued guidance supporting digital tools for informed consent, remote data capture, and safety monitoring.
4. Cost and Time Efficiencies:
Virtual trials often reduce costs by cutting down on site infrastructure and travel, while enabling faster patient recruitment across diverse populations. This shortens trial timelines accelerating drug time-to-market.
5. Rising Trial Volumes in Chronic Diseases:
As pharma companies race to develop new therapies, particularly in oncology, rare diseases, and cardiology, virtual and hybrid models offer scalable solutions for increasingly complex trials.
Regional Market Outlook
North America remains the largest market, commanding over half of global revenues. The U.S. leads thanks to high trial volumes, strong digital health adoption, and a regulatory landscape that supports innovation. Europe follows, with harmonized clinical trial regulations helping promote VCT adoption across member states.
Asia-Pacific is the fastest-growing region. Countries like China, India, and Japan are embracing digital trials due to their large patient populations, growing pharmaceutical industries, and supportive health-tech ecosystems.
Japan, in particular, is a market to watch. Though historically conservative, Japan’s VCT segment is accelerating as regulatory bodies adapt and local companies invest in modern digital trial platforms.
Key Companies Shaping the Market
IQVIA
ICON plc
Laboratory Corporation of America Holdings
Science 37
Parexel International Corporation
Oracle Corporation
Medidata Solutions
Signant Health
Veristat, LLC
Sanofi
Market Segmentation:
By Study Design: Interventional Trials, Observational Trials, Expanded Access Trials.
By Indication: Oncology, Neurology, Autoimmune/Inflammation, Cardiovascular Disease, Metabolic/Endocrinology, Infectious Disease, Ophthalmology, Others.
By Phase: Phase I, Phase II, Phase III, Phase IV.
By Region: North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
Latest News: USA
In the U.S., the landscape for virtual clinical trials continues to evolve rapidly:
Major pharma firms are expanding their decentralized trial programs, particularly in oncology and rare diseases areas where patient-centric design delivers clear advantages.
AI is being increasingly integrated into trial operations from predictive analytics for recruitment to adaptive trial designs that optimize protocols in real time.
Telemedicine providers are partnering with CROs and health insurers, creating seamless patient experiences across care and research.
The FDA continues to issue updated guidance on hybrid and decentralized models, providing greater clarity and encouraging broader adoption.
The U.S. remains the global leader in VCT innovation, thanks to a mature ecosystem of technology providers, CROs, and strong industry-regulator collaboration.
Latest News: Japan
Japan’s virtual clinical trials market, while smaller than the U.S. or Europe, is gaining momentum:
In 2024, the Japanese market was valued at approximately US$ 560 million, with steady growth expected through 2033.
Regulatory agencies in Japan are working closely with industry stakeholders to address long-standing barriers related to digital health and remote data collection.
Japanese CROs and pharma companies are entering strategic partnerships with global tech providers to deliver compliant, scalable VCT solutions.
Notably, leading firms have started implementing hybrid trial models in key therapeutic areas such as oncology, CNS (central nervous system), and metabolic disorders.
Japan’s aging population and growing burden of chronic disease are further incentivizing the use of digital-first clinical research approaches.
Overall, Japan’s VCT market is at a tipping point transitioning from pilot projects to broader-scale adoption as regulatory frameworks evolve.
Future Outlook
As the global life sciences sector increasingly embraces digital transformation, virtual clinical trials are poised to become more mainstream, complementing or even replacing traditional models in many cases. The benefits of this shift are substantial faster recruitment and higher patient retention rates streamline the trial process, while broader patient access ensures more diverse and representative study populations. In addition, virtual trials enhance overall efficiency and data quality through real-time digital monitoring and reduce operational costs by minimizing the need for physical infrastructure and travel. Together, these advantages are driving a fundamental evolution in how clinical research is conducted worldwide.
Looking For A Detailed Full Report? Get it here: https://datamintelligence.com/buy-now-page?report=virtual-clinical-trials-market
Purchase Your Subscription to Power Your Strategy with Precision: https://www.datamintelligence.com/reports-subscription
Related Reports:
Clinical Trial Supply & Logistics Market
Clinical Trial Management System Market
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.